FDA approves Probuphine, a six-month maintenance treatment
Death from opioid abuse – including heroin and prescription painkillers – is at an all-time high in the United States. But a new treatment option could be a “game changer” in the fight against opioid addiction, experts say.
Probuphine®, a tiny implant inserted into the arm, delivers a constant low dose of buprenorphine – a medication already used to treat opioid addiction – for six months.
The implant offers advantages over taking a daily pill or sublingual film of buprenorphine. Treatment compliance is far less of a challenge, since patients can’t skip, forget or abuse their medication. They also don’t have to deal with getting a prescription refilled. Some doctors say the implant is safer and easier to tolerate because it delivers even levels of buprenorphine into the bloodstream. The long-acting implant also reduces the risk of accidental ingestion by a child, and diversion of buprenorphine for illegal resale.
“For my patients who received the Probuphine, the overwhelming opinion was that it helped them feel more like a ‘normal person,’” says Michael Frost, M.D., one of the first physicians in the United States to administer Probuphine.
Frost, a board-certified addiction medicine specialist in Pennsylvania, was a principal investigator in the most recent clinical trial for Probuphine.
“None of my subjects relapsed during the course of the study,” says Frost. “They all universally felt that having the implants allowed them to focus less on the daily ritual/need to take medication and more on other aspects of their recovery and their lives such as family, career etc.”
78 Opioid-Related Deaths Daily:
Can Probuphine Ease the Toll?
Nationwide, just a handful of patients have received the new Probuphine implant, which was approved by the U.S. Food and Drug Administration (FDA) on May 26, 2016, after years of testing and debate.
Some experts raised concerns that the implant would deter patients from doing counseling, behavior therapy, or other changes needed to prevent relapse. At an FDA advisory hearing in January, others questioned whether buprenorphine pills would be required in addition to the implant, if the dosage was ineffective (Probuphine is intended only for patients already stable on low doses of oral buprenorphine). Another issue concerned potential relapse after the six-month implant is removed, and how to get back on track.
But after reviewing the clinical evidence – and hearing testimony from addiction experts and people in recovery – the FDA advisory panel voted 12-5 to recommend approving Probuphine. Health officials hope the implant’s long-acting effects can blunt the nation’s epidemic of opioid abuse.
“Probuphine has the potential to be a game-changer in how we address the opioid crisis,” says Jack Stein, Ph.D., Director of the Office of Science Policy and Communications at the National Institute on Drug Abuse.
An estimated 78 people die each day in the United States from opioid overdose, and fatalities from all drug overdoses are now the leading cause of injury death in America, surpassing car accidents. Drug overdoses claimed the lives of 47,055 Americans in 2014 – more than any previous year on record – and opioids accounted for 61 percent of those deaths, according to the U.S. Centers for Disease Control and Prevention (CDC).
Stein says the implant adds “yet another effective medication to the clinical toolbox, particularly among patients where compliance may be a challenge such as those involved in the criminal justice system.”

People with opioid use disorders can receive the Probuphine implant at a doctor’s office; the entire procedure takes about 15 to 20 minutes. But finding a physician to administer Probuphine is challenging, until more providers are approved. Doctors are required to be specially trained and certified in the procedure, since the implant must be surgically inserted and removed.
To date, more than 1,100 physicians in 44 states have been certified to provide treatment with Probuphine (mostly addiction medicine specialists and psychiatrists), according to Braeburn Pharmaceuticals. The company owns commercial rights to Probuphine in the United States and Canada under a 2012 license agreement with Titan Pharmaceuticals.
Braeburn estimates that more than 4,000 U.S. doctors will be trained in the implant procedure by the end of 2016.
A Probuphine provider list can be seen at http://probuphinerems.com/probuphine-locator/.
Implant Candidate:
Stable, Already In Treatment
Not everyone is a candidate for the implant. Probuphine is intended for maintenance treatment of drug addiction; in other words, opioid users who are already stable on low-dose buprenorphine.
“It is important to understand that the implant is approved only for individuals with opioid dependence who have already been treated with, and are medically stable on, existing orally absorbed buprenorphine formulations,” Stein says, “thus giving physicians a valuable new therapeutic tool for this subset of patients.”
Probuphine is the only implantable treatment for opioid dependence, and was developed using proprietary technology from Titan Pharmaceuticals. That technology – known as ProNeura™ – is a drug delivery platform that consists of a slender rod (the implant) made from a mixture of ethylene-vinyl acetate and a drug substance (buprenorphine in this case). The delivery system releases the medication from under the skin, slowly and continuously – similar to how intravenous drugs are administered.
Researchers at the Massachusetts Institute of Technology (MIT) developed the implant concept; Titan licensed the patent from MIT in 1996.
“The implant technology allows the formulation to release the drug slowly over an extended period of time, without the up-and-down drug levels in the blood that happen with oral formulations,” says Kate L. Beebe, Ph.D., Executive Vice President and Chief Development Officer at Titan Pharmaceuticals.
The Evidence on Probuphine
Probuphine implants have been tested since early 2000, Beebe says. Clinical trials began with a small study in Australia, since the FDA had not yet approved buprenorphine (in any form) for treating opioid addiction in the United States.
 “The study that we did was in 12 adults who were chronic heroin users,” Beebe says. “The results were that these implants were able to release constant, low levels of the drug consistently over six months – and the implant effectively controlled patients’ withdrawal symptoms and opioid cravings.”
“The study that we did was in 12 adults who were chronic heroin users,” Beebe says. “The results were that these implants were able to release constant, low levels of the drug consistently over six months – and the implant effectively controlled patients’ withdrawal symptoms and opioid cravings.”
Both heroin and prescription drug users were enrolled in the first U.S. study of Probuphine, published in the Journal of the American Medical Association (JAMA) in October, 2010. In that study, 163 opioid-dependent adults were either given the Probuphine implant or a placebo implant for six months. Then urine tests were conducted to determine opioid use.
“There was a more significant reduction in opioid use with people who were treated with the implant, compared to placebo,” Beebe says.
Specifically, the study showed that 40.4 percent of the implant patients tested negative for illicit opioids at four months, compared to 28.3 percent of the placebo group.
Patients who received the real Probuphine implants also had fewer withdrawal symptoms, less drug cravings, and greater change on clinical measures of addiction severity, according to the study results. Patient retention was also higher: 65.7 percent of patients on Probuphine were able to complete the six-month study, vs. only 30.9 percent of the placebo group (many dropped out because of opioid cravings and withdrawal symptoms).
A more recent study compared the effectiveness of the Probuphine implant with the current standard of care for opioid dependence: sublingual tablets containing a combination of buprenorphine and naloxone (brand name: Suboxone).
The six-month study, conducted by Braeburn Pharmaceuticals in consultation with the FDA, tested 177 clinically stable patients who received either Probuphine or the sublingual Suboxone tablet. Results showed that 88 percent of the implant patients tested negative for opioid use for the six months, vs. 72 percent of patients on sublingual tablets. Opioid cravings and withdrawal symptoms were comparable in the two groups, and the implant insertion and removal was “generally well-tolerated,” the study reported (23 percent of Probuphine patients had what clinicians deemed a “mild” reaction at the implant site).
“One key advantage of Probuphine over a daily pill is giving an individual who’s fighting this disease freedom from having to think about taking a pill every day,” says Beebe. “For people who are really struggling with addiction, situations can happen where they’re in the wrong place at the wrong time and there’s temptation to use. (Probuphine) gives them protection against that.”
The Probuphine Implant: A Closer Look
What is the Probuphine implant and how does it help opioid addiction?
Probuphine is the first implant designed to treat addiction to opioids such as heroin or prescription narcotics. Multiple clinical trials have demonstrated that the implant, approved by the FDA in May, is effective at reducing opioid cravings and withdrawal symptoms.
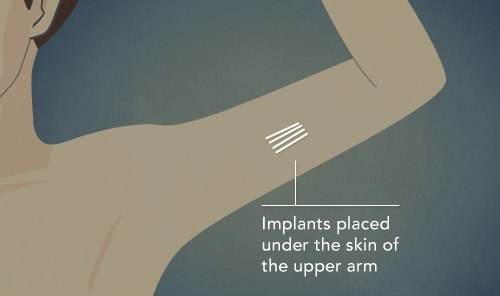
The implant is inserted into the upper arm and delivers a constant, low dose of the medication buprenorphine for six months at a time.
Oral forms of buprenorphine have been used to treat opioid dependence for more than a decade. Buprenorphine works by binding to opioid receptors in the brain, which prevents debilitating withdrawal symptoms when someone stops taking opioid drugs.
Who is a candidate for Probuphine?
Patients with opioid use disorders who are already stable on low-to-moderate doses of oral buprenorphine (8 mg. or less daily) are candidates for the implant. This is a long-acting maintenance treatment and is not appropriate for those just entering addiction treatment.
How much does the Probuphine implant cost? Is it covered by insurance?
The cost of the Probuphine implant is $4,950 for a six-month course of treatment.
Braeburn Pharmaceuticals, which oversees the U.S. commercial launch of Probuphine, says insurers have expressed “strong interest” in discussing coverage for Probuphine. Braeburn notes that United Healthcare and several Blue Cross/Blue Shield plans approved insurance reimbursement for the first patients implanted.
“Now that the FDA has approved Probuphine, Braeburn’s top priorities are to train and certify healthcare providers to make Probuphine available to patients across the country and to establish insurance coverage as quickly as possible,” Braeburn President and CEO Behshad Sheldon said in a statement.
How big is the implant and what happens during the insertion procedure?
The Probuphine implant consists of four slender rods, about the size of a matchstick. Each implant delivers the equivalent of 80 mg of buprenorphine over six months.
A specially trained physician inserts the implant, typically on the inside of the upper arm, using a local anesthetic. This can be done in an office visit and takes 15 to 20 minutes.
A patient may experience mild tugging or pulling as the implant is inserted (or removed). There may be some pain or swelling initially but “typically patients tolerate this very well,” says Kate Beebe, Ph.D., Executive VP and Chief Development Officer at Titan Pharmaceuticals. “In our clinical trials, it was not a deterrent for people wanting to receive future treatment.”
What are the risks/side effects?
As with other forms of buprenorphine, side effects of the implant in clinical trials included the following symptoms: headache, insomnia, upper respiratory tract infection, nausea, anxiety, back pain, depression, constipation, and vomiting.
Patients may also have a reaction at the implant site, such as pain, itching and redness. Rare but serious complications including nerve damage or potentially fatal embolism could result from improper insertion. Due to those risks, Probuphine can be administered only by physicians who complete a live training program on the insertion and removal procedures and become certified before performing the procedure.
What happens after the six-month treatment period ends?
After six months, the implant is removed (it’s not biodegradable). If further intervention is needed, new implants may be inserted in the opposite arm for an additional course of treatment. The FDA is requiring studies on the long-term use of Probuphine.
How do I get the Probuphine implant?
Probuphine will not be available in local pharmacies; the implant can only be prescribed and administered by a trained and certified physician. Braeburn Pharmaceuticals is working to train at least 4,000 doctors in this treatment option by the end of 2016.
To locate a Probuphine provider, go to: http://probuphinerems.com/probuphine-locator/
What else do I need to do for my recovery?
Opioid addiction is a chronic, relapsing brain disease that requires vigilance to prevent relapse. The Probuphine implant is intended to be part of a comprehensive treatment program that includes a combination of medication, psychosocial support and counseling.


